Valence Shell Electron Pair Repulsion Theory
Valence shell electron pair repulsion theory, VSEPR, is a super-simple technique for predicting the shape or geometry of atomic centres in small molecules and molecular ions:
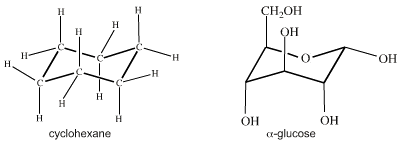
Crucially, atomic centres with VSEPR determined geometry can be joined together into molecular entities like cyclohexane and glucose:
This molecular building-block logic can be extended, enabling large biomolecular structures like DNA to be modelled and understood:
This page is concerned with the geometries of ligands about single atomic centres, and to help this understanding there are a series of drills associated with this page to test your knowledge.
Searches for real chemical entities based on VSEPR geometry can be made using the web based Chemical Thesaurus reaction chemistry database [also by meta-synthesis].
The CoolMolecules Molecular Structure Explorer website classifies molecules by the shape/geometry of the central atom, and all structures are all obtained from experimental data. Searches on the extensive database can be made by atom, shape, experimental method and molecules can be rotated. Highly recommended.
The VSEPR Technique
Six or so steps are required to generate the VSEPR geometry of an atomic centre such as:
- Carbon in methane, CH4
- Nitrogen in ammonia, NH3
- Xenon in xenon tetrafluoride, XeF4
- Iodine in the iodide difluoride ion, [IF2]–
First: Determine the number of electrons in the outer (valence) shell about the central atom (C, N, Xe, I, etc.): 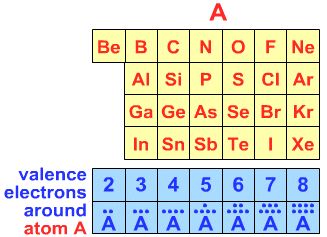 Carbon, for example has four valence electrons, nitrogen 5, etc. |
|
Second: Find valency and number of electrons associated with the ligand X:
|
|
Third: Construct a valid Lewis structure of the molecule in question showing all of the bonds and all of the lone pairs (nonbonded pairs) of electrons. If the structure is a molecular ion, add one valence electron for each negative charge and remove one valence electron for each positive charge. 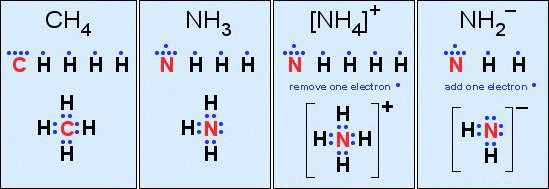
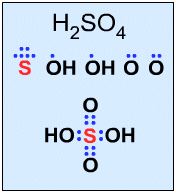
Not all Lewis structures have eight electrons about the central atom A (as emphasised by very simple Lewis octet theory). For example, Sulfuric acid, H2SO4, has two monovalent OH functions and two doubly bonded oxygens that behave as single ligands:
Phosphorus pentachloride, PCl5, has 10 electrons: 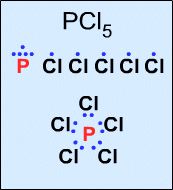 |
|
Fourth: Determine the "total coordination number" of the central atom, where:
|
|
Fifth: The overall geometry of the atomic centre is determined by the mutual repulsion between the electron pairs of the total coordination number.
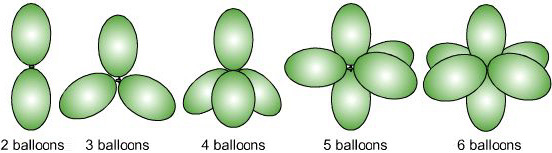
|
|
Sixth: There two adjustments are required by the VSEPR method to find the geometry of an atomic centre:
Lone-pairs of electrons (blue) behave as if they are slightly bigger than bonded electron pairs (green) and act to distort the geometry about the atomic centre so that bond angles are slightly smaller than expected: Methane, CH4, has a perfect tetrahedral bond angle of 109° 28' (109.47°), while the H-N-H bond angle of ammonia, H3N:, is slightly less at 107°: 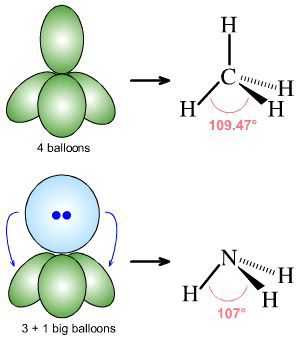 |
By Starjester1 as a Honors Chemistry class project:
The AXE system
American General Chemistry textbooks – but for some reasom not British ones – adopt the excellent AXmEn system, where A is the central atom, m the number of ligands X, and n the number of nonbonded lone-pairs of electrons, E, about the central atom.
In this system:
Methane, CH4, is AX4
Ammonia, H3N:, is AX3E1
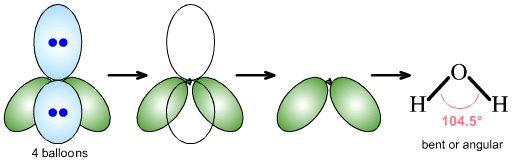
water, H2O, is AX2E2
Note that different AXmEn designations can give rise to the same overall geometry or shape:
For example:
AX2E1 and AX2E2 both give rise to bent or angular geometries
AX2 and AX2E3 both give rise to linear geometries
Patterns in AXE Space
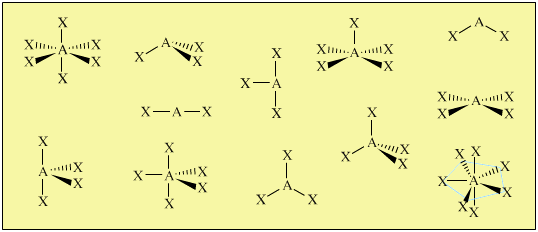
The AXE system gives rise to a pattern, from which the various atomic geometric shapes can be determined/assigned:
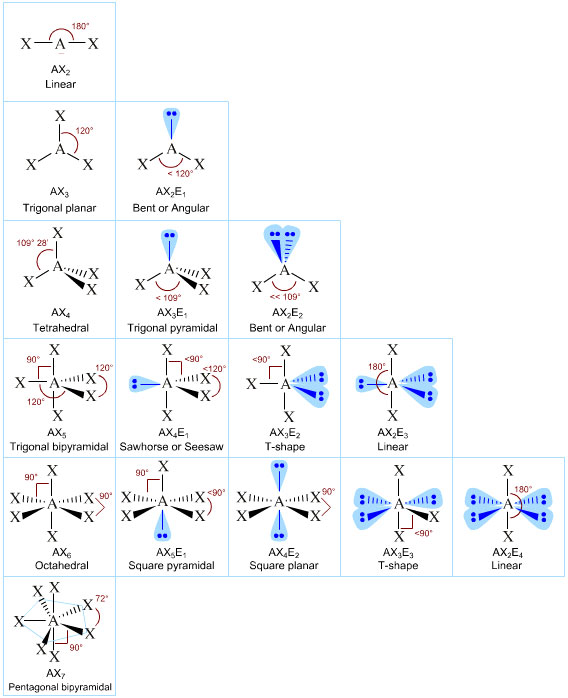
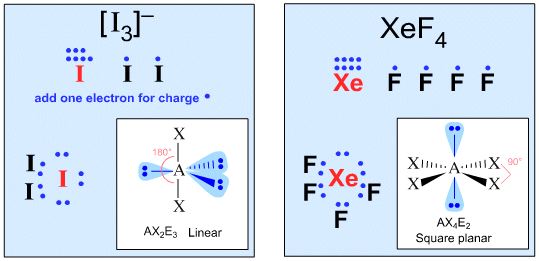
A Couple of More Advanced Examples:
Students: What You Need To Know
To be proficient in chemistry at the university entrance level [American AP, British AS/A2 or French Baccalaureate] it is absolutely essential to be able to recognise the VSEPR geometries, know the associated names and work out VSEPR structures from formula of the AEX systems listed below:
AX2
AX3
AX4
AX5
AX6
AX3E1
AX2E2
At university level, all of the atomic centre geometries (plus associated point groups) must be known: